Sponsored – Est. Read 9 Min
Massive Disruption Ahead for Multi-Billion-Dollar Mental Health Treatment Market
One Under-the-Radar Company – with Over 20 Issued Patents – Appears to Be the Only One Capable of Offering Naturally-Derived Psychedelic Drug Candidates to Treat Devastating Mental Health Conditions
Here’s why investors should pay close attention to what’s happening with Filament Health Corp. (NEO: FH); (OTC: FLHLF) right now.
There’s no question that we are in the midst of a significant global mental health crisis.
Virtually every adult either knows someone or has a family member who has struggled with substance use or end-of-life distress.
These are terrible problems…and unfortunately millions of people continue to suffer from them because effective treatments have so far been elusive.
One little-known psychedelic drug developer, Filament Health Corp. (OTC: FLHLF); (NEO:FH) is working to change that through naturally-derived psychedelic medicines that can help treat those suffering from mental health conditions.
Filament Health Corp. is a clinical-stage natural psychedelic drug development company working to develop natural treatments for some of the world’s most difficult health problems.
“The Next Big Addiction Treatment: A growing body of data points to one psychedelic drug as the leading contender to treat the intractable disease of substance abuse: Psilocybin, the active ingredient in psychedelic mushrooms.”

“Wall Street is betting tens of millions of dollars on psychedelic drugs that…could treat mental illness for a fraction of what it costs to do therapy with better-known treatments.”

So what makes the Filament Health story so unique?
Filament Health Corp. was founded by a team of experts in botanical extraction and is the first – and so far, the only – company that has figured out how to make pharmaceutical grade natural psychedelics.
Prior to Filament Health Corp., nobody had ever successfully taken a natural psychedelic drug product into a clinical trial.
The majority of today’s more well-known psychedelic drug developers only create synthetic psychedelic compounds because synthetic manufacturing of isolated compounds is much easier than extracting and standardizing botanical drugs which contain all of the active compounds from a natural source.
Thanks to its founders’ proven history of success with extraction, Filament Health Corp. is now emerging as a leading supplier of psychedelics either natural or synthetic.

For investors, Filament Health Corp. (OTC: FLHLF); (NEO:FH) now offers smart exposure to the rapidly growing psychedelics market as the company represents a unique combination of its own proprietary extraction technology as well as the potential superiority of natural psychedelics over synthetics.
Now here are seven key reasons why you should take a close look at Filament Health Corp. (OTC: FLHLF); (NEO:FH) and consider adding it to your portfolio:
7 Key Reasons
Why You Should Strongly Consider the Upside Potential for Filament Health Corp. (OTC: FLHLF); (NEO:FH)
Key Reason #1: Massive, Multi-Billion-Dollar Addressable Market
Filament Health Corp. believes that standardized, naturally-derived psychedelic medicines can improve the lives of millions of people suffering from treatable mental health and substance-use conditions.
To that end, the company’s primary focus is on developing naturally-derived psychedelics to help treat stimulant use disorder and opioid use disorder.
This represents a potentially massive, multi-billion-dollar market opportunity for the company.
According to the experts at Transparency Market Research, the global substance abuse treatment market is projected to grow from US $10.2 billion as recently as 2021 to more than US $23.1 billion by 2031.iii
This market growth is taking place even though existing treatments can be woefully inadequate or non-existent. In fact, as many as 40-60% of individuals relapse within 30 days of leaving an inpatient drug and alcohol treatment center, and up to 85% relapse within the first year.[iv]
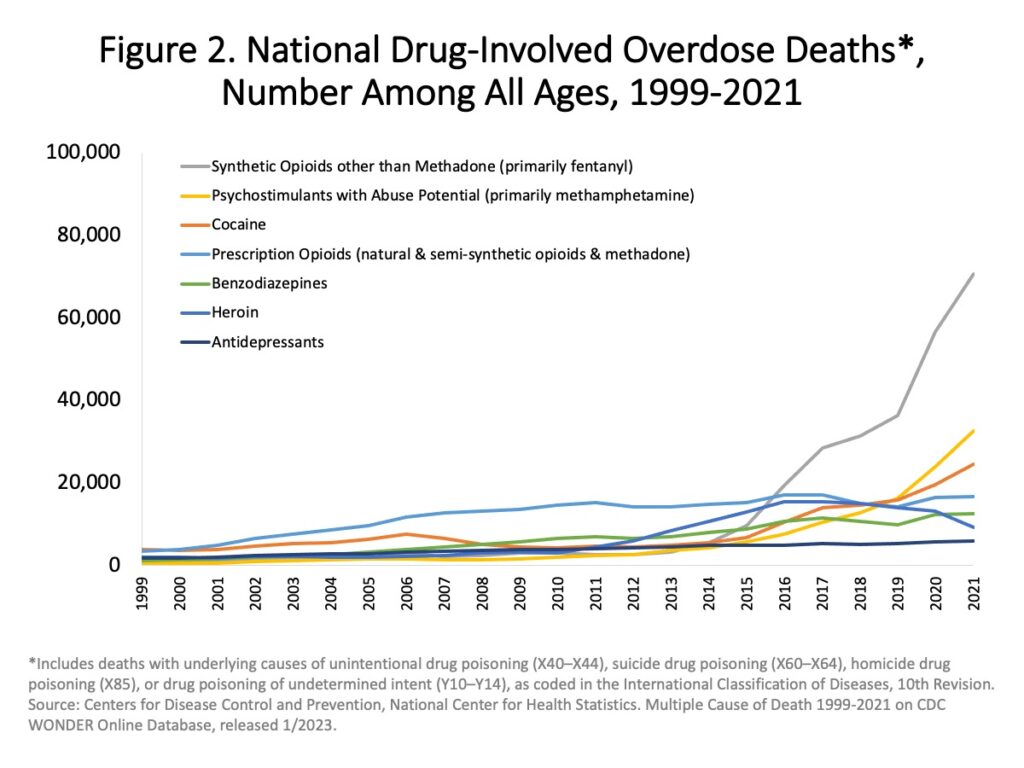
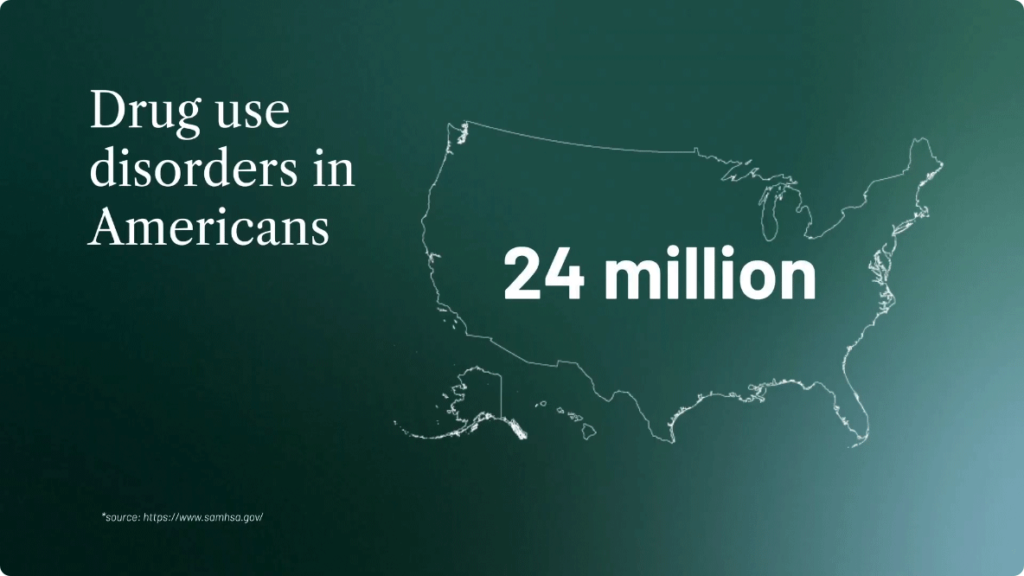
Here are just a few key measures of how massive the substance use disorder problem has become:
- Among Americans aged 12 or older in 2021, 8.6% — or 24 million people – had at least one drug use disorder in the past year.[v]
- In the latest national survey, conducted in 2021, 9.2 million Americans reported opioid misuse and 5.6 million reported an opioid use disorder (OUD).[vi]
- In that same national survey, 11 million Americans reported stimulant misuse and 4.5 million reported a stimulant use disorder (StUD).[vii]
- And from 2012 through 2019, the fatal overdose rate increased on average by 295 per year to 5.0 deaths per 100,000 standard population. At the same time, opioid overdose deaths nearly doubled from 7.4 5o 15.5 deaths per 100,000.[viii]
This rapidly growing challenge of substance use disorder has become one of the world’s most pressing public health issues.
Filament Health Corp. (OTC: FLHLF); (NEO:FH) is working to meet this challenge – and address this large market – by doing something no other company is currently capable of: Delivering naturally-derived psilocybin treatments as part of a comprehensive therapy.
As of today, Filament Health Corp. (OTC: FLHLF); (NEO:FH) is the only company in the world whose natural psychedelic drug candidates have been authorized by regulatory bodies such as the FDA and Health Canada for administration in human clinical trials.
BREAKING NEWS
Filament Health Announces Signing Of Definitive Agreement for Convertible Note Financing, Closing of C$900,000 Non-Brokered Private Placement Financing, Founder-Led Note Offering, And Other Updates Related To Proposed Business Combination
On December 6, 2023 Filament Health Corp. announced that it had entered into a definitive securities purchase agreement with Helena Global Investment Opportunities 1 Ltd., providing for up to US $14.4 million in funding.
Key Reason #2: Drug Candidates Derived from Natural Sources
Filament Health Corp. is unique in the psychedelic drug development space because the company believes strongly that nature – not a lab – is the best source for psychedelic drug candidates. This is the way humans have always found new medicine, especially in terms of psychedelics.
Filament Health has developed proprietary technology for manufacturing botanical drugs from natural psychedelic sources. This allows Filament Health Corp. to leverage nature’s historical evidence and provides a faster path to human clinical trials.

In fact, Filament Health Corp. is the first company to get a naturally-derived psychedelic into a clinical trial.
Filament’s drug development pathway offers the best of both worlds by combining the benefits of well-known substances with robust IP protection. That’s because the complexity of the formulation with botanical drugs makes Filament’s products extremely difficult – if not impossible – to genericize.
The company’s platform offers the potential for hundreds of drugs to be discovered and run through Filament’s in-house development program or through its partnership network.
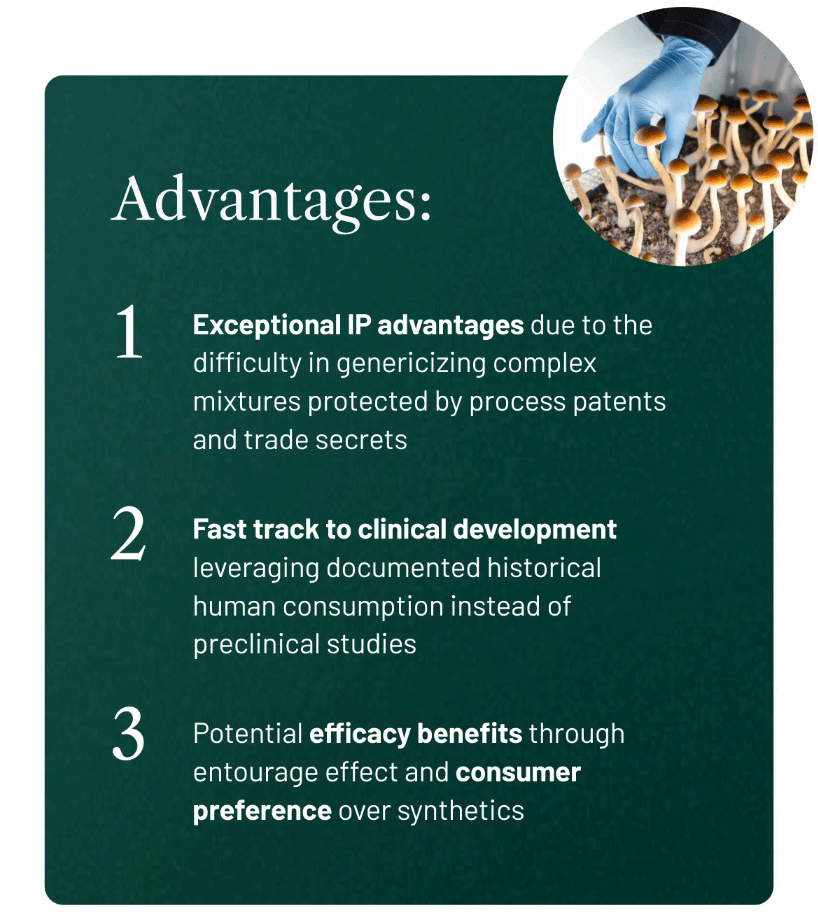
And naturally-derived psychedelics offer the potential for FDA “fast-track” development, which provides an expedited and more cost-effective route to the clinic.
This special consideration where botanical drugs resemble a substance used by humans allows for an accelerated move toward clinical trial saving years of development time and millions of dollars in development costs.
Another advantage of naturally-derived botanical psychedelics is that these natural drugs contain all of the secondary metabolites from the raw material whereas synthetics do not.
Of course, magic mushrooms contain much more than just psilocybin. These secondary compounds are part of the API and may improve the efficacy of the treatment via the entourage effect.
Filament Health Corp.’s focus on natural psychedelics preserves the connection to the way humanity – for thousands of years – has always consumed these substances.
Modern consumers reflect this preference by seeking out natural products whenever possible, leading to the popularity of natural food and wellness brands as well as stores such as Whole Foods.
Key Reason #3: Highly Promising Drug Candidates Already in Clinical Trials
The team at Filament Health Corp. has used its botanical extraction expertise to develop what are believed to be the first-ever standardized, natural psychedelic drug candidates.
The team at Filament’s Health Canada-licensed, GMP-compliant facility in metro Vancouver has created standardized drug candidates extracted from magic mushrooms, iboga roots, San Pedro cacti and more.
Filament Health Corp.’s PEX010 is a botanical psilocybin drug candidate that is now being studied in clinical trials all over the world for indications including:
- depression
- alcohol use disorder
- anxiety
- opioid tapering.
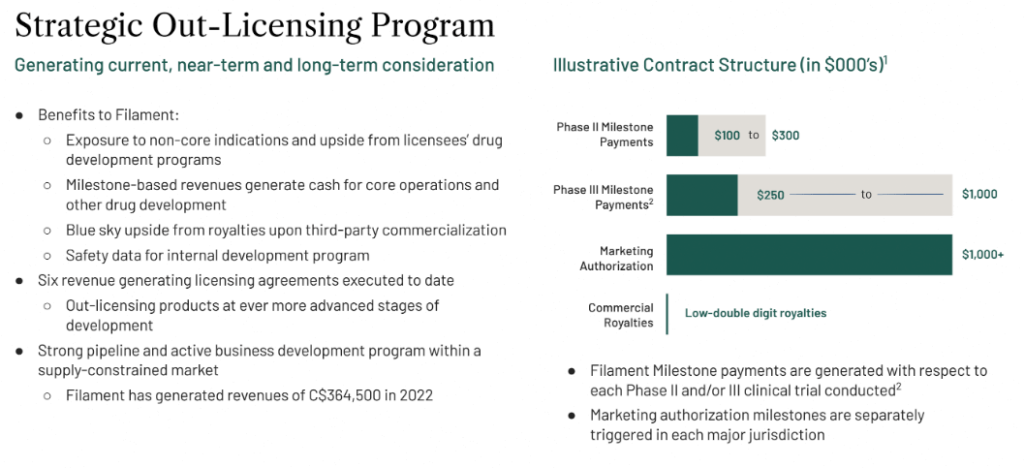
Filament Health Corp. out-licenses PEX010 to other commercial entities, allowing the company to generate licensing and supply revenue.
Filament Health Corp. has also built a network of academic and research partners that includes leading institutions such as the Canadian Association for Mental Health, the University of Toronto, UCLA and the University of Copenhagen.
* In August 2023, the company announced approval from the United States Food and Drug Administration for two clinical trials using PEX010, at Washington School of Medicine for the treatment of cancer-related anxiety and at the University of California, Los Angeles studying effects of joining psilocybin treatment with cognitive-behavioral therapy for patients with depression.
* And in July 2023, the company announced that it will supply psilocybin for two clinical trials that received The Canadian Institutes of Health Research Operating Grants for Psilocybin-assisted Psychotherapy for Mental Health and Substance Use Disorders. The clinical trials will study the effects of Filament’s botanical psilocybin drug candidate, PEX010, for alcohol use disorder and treatment-resistant depression.
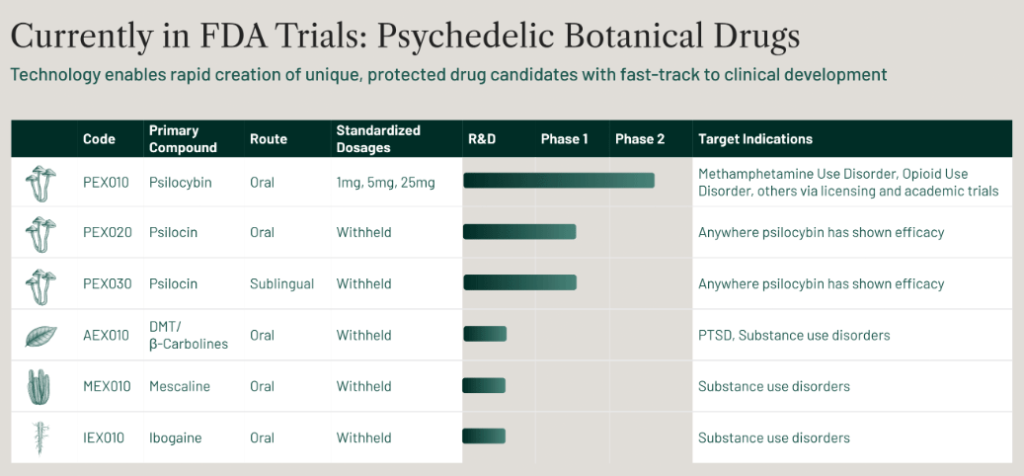
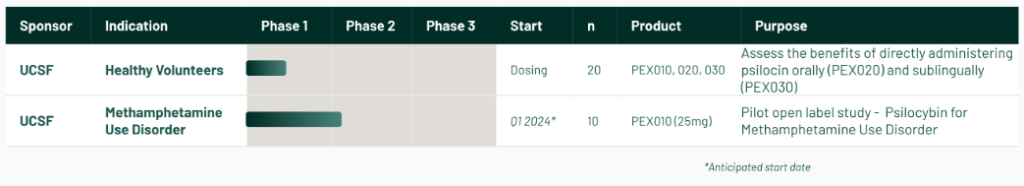
The company’s psilocin drug candidates PEX020 and PEX030 are currently being studied at the University of California San Francisco’s translational psychedelic research program in a Phase 1 clinical trial.
This is the first ever FDA-approved clinical trial of naturally-derived psychedelic drugs.
In addition, the FDA has approved a Phase 2 trial also at UCSF studying PEX010 for methamphetamine use disorder.

Key Reason #4: Filament Health Corp. is Moving Quickly to Build Shareholder Value
In a relatively short time, the team at Filament Health Corp. (OTC: FLHLF); (NEO:FH) has accomplished a great deal.
For starters, the company has established a strong portfolio of intellectual property.
As of September 2023, the company has 20 issued patents and at least 45 additional patent applications believed to create significant barriers to entry.
Importantly for investors, this significant intellectual property portfolio helps create a large moat for Filament Health Corp.
Simply put, there are a finite number of ways to extract, purify and standardize naturally-occurring psychedelic compounds.
And Filament Health Corp. (OTC: FLHLF); (NEO:FH) is leading the way and establishing critical patent protection for many of those methods.
The company has already established diversified revenue streams and is able to license its IP, outlicense its drug candidates, and provide contract manufacturing services. As one of the only psychedelic drug developers currently generating revenue through its commercial partnerships, the company is able to support its operations while mitigating dilution.
Additionally, since the company went public after the “boom” in the psychedelics market of 2020-21, the company has done more than its competitors…with significantly less.
While other companies in the psychedelics space have raised $100 million or more to date, Filament Health has raised just $15 million.
In fact, the company’s current market cap of just under US $28 million is greater than the total amount of money it has raised to date.
…and this is all in service of its larger goal of developing pharmaceutical grade naturally-derived psychedelics to help treat many of the world’s most challenging mental health conditions.
Key Reason #5: The Experts Behind Filament Health Have Already Successfully Built a Natural Extraction Startup
When considering any small cap investment, it’s critical to examine the company’s leadership team and their history of success in the industry.
When it comes to Filament Health Corp. (OTC: FLHLF); (NEO:FH) you’ll find a team of proven experts in the extraction space who have already helped build – and successfully exit – one extraction startup.
And now they stand poised to do it again.
The leadership team at Filament Health honed its expertise at Mazza Innovation, a natural extraction startup which many of the team now at Filament helped scale from pre-seed to an exit in 2018.
At Mazza, this team developed and commercialized patent-protected extraction technologies at a 35,000 ft2 commercial-scale GMP facility.
Having helped scale Mazza to such impressive heights, Mazza was acquired by Sensient Technologies – a $3 billion NYSE company for $26 million – which was 26 times revenue at the time.
After selling Mazza, Filament’s CEO and Co-Founder Ben Lightburn took note of the growing global mental health crisis.

Ben Lightburn is a proven entrepreneur and leader specializing in the research, development and commercialization of novel extraction technologies.
He believes strongly that psychedelics could help those struggling with mental health, and that natural psychedelics will be the preferred choice.
Ben also recognizes his unique position, as he possesses the skills and experience to develop natural psychedelic drugs. By combining his background as entrepreneur with the botanical extraction expertise of the former Mazza Innovation team, Filament Health was created.
Seven of the team members at Filament Health, including Ben Lightburn, were part of the team at Mazza, including:

Lisa Ranken – Chief Operating Officer, P.Eng., M.Eng.
Operational and leadership expertise in rapidly growing industries; Ms. Ranken was VP of Operations at Mazza Innovation.

Ryan Moss – Chief Science Officer – M. Analytical Chemistry
Expert in the field of botanical extraction, purification, standardization; Mr. Moss was Director of Research at Mazza Innovation.

Andry Tjahyana – Vice President, Business Development – BBA
Mr. Tjahayana has over 25 years of experience in B2B business development and is a former senior executive of several pharmaceutical and natural product organizations, including his role as VP of Business Development at Mazza Innovation.

Beatriz Ramons – Director of Quality – B.Sc
Quality management, regulatory compliance, and attaining third-party certification; Ms. Ramos was Manager, Quality Assurance at Mazza Innovation.
Key Reason #6: Vertically Integrated, In-House GMP Manufacturing
Filament Health’s vertically integrated, in-house GMP manufacturing allows for rapid product and IP development and establishes timely fulfillment of partner requests.
The company’s current production capacity is 2500 high doses of psilocybin per month.
Filament Health’s research and development facility is one of the first in the world to hold a Health Canada Dealer’s License and be GMP-compliant. These capabilities are required to manufacture and distribute drugs for human consumption in clinical trials or via authorized compassionate use.
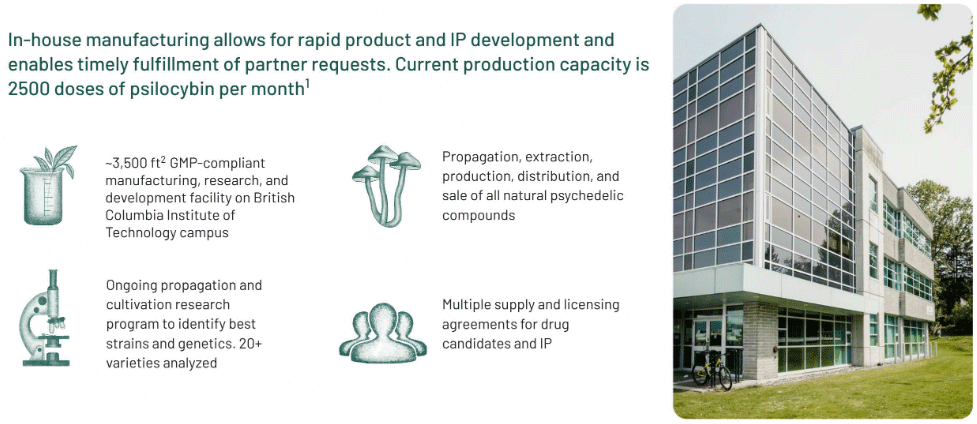
Filament Health has developed technology which facilitates the production of stable, standardized formulations of any psychedelic species.
These innovations have yielded patented methods to produce the world’s first pharmaceutical-grade, botanical psychedelic drug candidates, which are being studied in clinical trials around the world
Key Reason #7: Filament Health Corp. (OTC: FLHLF); (NEO:FH) Has the Potential for Significant Growth
So just what type of potential could a company like Filament Health Corp. (OTC: FLHLF); (NEO:FH) offer?
In looking at other publicly traded psychedelic companies, it seems clear there is plenty of room for growth in valuation.
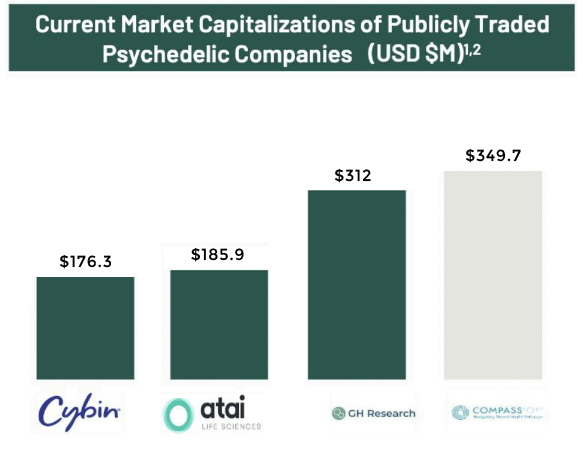
For example, a company such as Atai Life Sciences N.V. (Nasdaq: ATAI) is a clinical-stage biopharmaceutical company specializing in treatment for mental health disorders and it currently has a market cap of USD $185.9 million.
GH Research (Nasdaq: GHRS) is another clinical-stage biopharmaceutical company focused on treatment of psychiatric and neurological disorders…and currently has a market cap of US $312 million.
And COMPASS Pathways plc (Nasdaq: CMPS) – another psychedelic company developing treatments for mental health – is currently valued at a market cap of US $349.7 million.
Meanwhile by comparison, Filament Health Corp. (OTC: FLHLF); (NEO:FH) – with its significant IP portfolio and its natural psychedelics already in clinical trials, and network of customers – currently has a market cap below each of these competitors…with plenty of room to run.
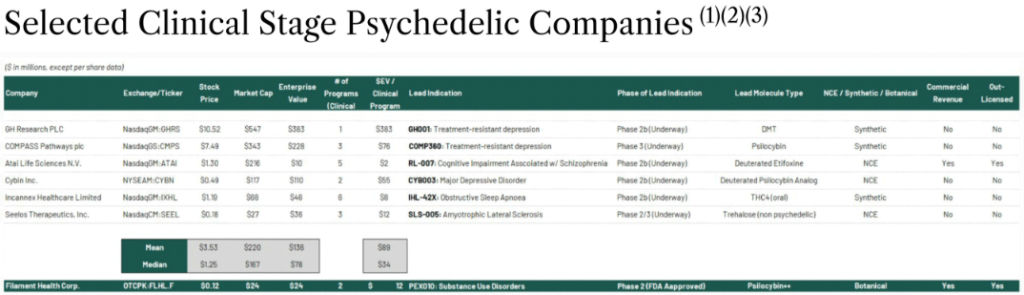
Since its inception in 2020, Filament Health Corp. has been laser-focused on establishing its manufacturing process and building its IP portfolio.
With multiple drugs now in clinical trials – and as the only company whose natural psychedelic drug candidates have been authorized for human trials – Filament Health’s story could spread rapidly…leading to a potential significant change in its valuation.
Investor’s Summary
Filament Health Corp. (OTC: FLHLF); (NEO:FH) offers investors exposure to the massive, multi-billion-dollar market for mental health treatment.
As psychedelics become a more proven and popular treatment option, Filament Health Corp. has emerged as the only company capable of developing pharmaceutical grade, natural psychedelic drug candidates.
With a wide moat of intellectual property – and a low valuation as the company flies beneath Wall Street’s radar – Filament Health Corp. (NEO: FH); (OTC: FLHLF) now offers investors significant upside potential.
i https://www.nytimes.com/2022/03/31/well/mind/psilocybin-
mushrooms-addiction-therapy.html
ii https://www.wsj.com/articles/wall-street-backs-
new-class-of-psychedelic-drugs-3c5b9baf
iii https://www.transparencymarketresearch.com/
substance-abuse-treatment-market.html
iv https://www.familyaddictionspecialist.com/blog/
10-most-common-reasons-for-addiction-relapse#:~:
text=Unfortunately%20relapse%20rates%20for
%20individuals,relapse%20within%20the%20first%20year.
v https://www.samhsa.gov/data/sites/default/files/reports/
rpt39443/2021NSDUHNNR122322/2021NSDUHNNR122322.htm#
vi https://www.samhsa.gov/data/sites/default/files/reports/
rpt39443/2021NSDUHNNR122322/2021NSDUHNNR122322.htm#
vii https://www.samhsa.gov/data/sites/default/files/reports/
rpt39443/2021NSDUHNNR122322/2021NSDUHNNR122322.htm#
viii https://www.cdc.gov/nchs/products/databriefs/db394.htm
Disclaimer:
