This Unknown Company is Unlocking the Power of Cannabinoids for the Treatment of Serious Neurological and Inflammatory Disorders

(OTCBB: VBIO)
VBIO – DEVELOPING REVOLUTIONARY TREAMENTS FOR NEUROLOGICAL AND INFLAMMATORY DISORDERS SUCH AS INFLAMMATORY BOWEL DISEASE AND MULTIPLE SCLEROSIS

Vitality Biopharma is a cure development company, dedicated to unlocking the power of cannabinoid ‘prodrugs’ as a means to treat serious neurological and inflammatory disorders.
Vitality Biopharma’s mission is to improve the lives of patients afflicted with devastating neurological and inflammatory conditions, such as multiple sclerosis.
Vitality Biopharma goes above and beyond providing relief to patients afflicted with these disorders, treating muscle spasticity in multiple sclerosis and identifying combination treatments that treat the underlying cause of disease and hold the potential to help patients recover lost function
Vitality Biopharma has filed intellectual property applications including strong composition of matter claims for more than 30 cannabinoid prodrugs, including versions of THC, CBD, and CBDV, and has demonstrated the ability to create a proprietary prodrug of every significant cannabinoid pharmaceutical available today.
Cannabinoid Overview
- The pharmacology of cannabinoids provides significant potential for therapeutics across several diseases that
 form the core of specialty pharmaceutical drugs.
form the core of specialty pharmaceutical drugs. - Revenues in the cannabis biotech/pharmaceutical sector could represent 10% of the overall specialty pharmaceutical market over the next five years,
- Potential market size of at least $20 billion.
- Cannabinoids are a class of more than 60 molecules found within Cannabis sativa, and they are the active ingredients in medical marijuana.
- Due to federal restrictions, there traditionally has been a lack of pharmaceutical research dedicated to exploring their medical claims, which has led some to describe cannabinoid research as ‘a neglected pharmacological treasure trove’.
- Cannabis exerts pleiotropic (producing more than one influence; exhibiting multiple expressions) effects on the brain, and medical marijuana has been prescribed for applications ranging from chronic pain, nausea/vomiting, severe anorexia, and muscle spasticity.
- Clinical studies have evaluated effects on chronic pain, neuropathic pain, multiple sclerosis, and many other neurological disorders, with success.
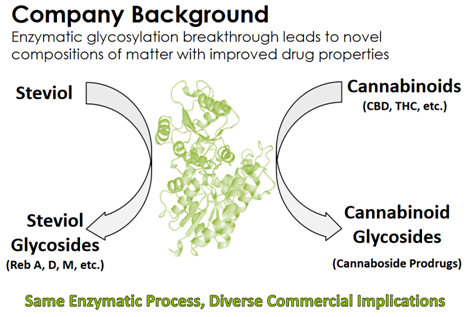
VBIO recently announced it had developed a new class of cannabinoid prodrugs, known as cannabosides,
Upon ingestion, these cannabosides can enable the selective delivery of THC and cannabidiol (CBD) to the gastrointestinal tract.
VBIO believes site-specific delivery could enable oral drug formulations of cannabinoids to provide therapeutic benefits while reducing or avoiding the systemic delivery of THC into the bloodstream. Currently, high concentrations of psychoactive THC in the brain limit the dose of cannabinoids that can be used elsewhere in the body for treatment of pain and inflammation.
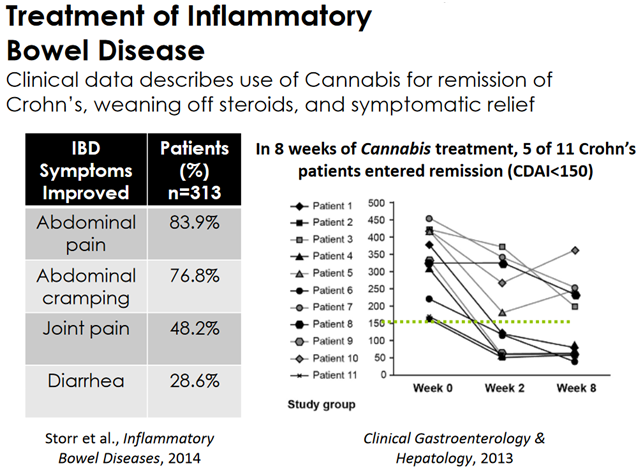
Independent clinical trial results suggest that cannabinoids will help induce remission in Crohn’s disease patients.
The vast majority of inflammatory bowel disease patients experience symptomatic relief, including more than 75% of patients who report improvement in visceral pain and abdominal cramping.
Approximately 1.4 million Americans are affected by inflammatory bowel diseases, including Crohn’s disease and ulcerative colitis. Most patients are diagnosed before age 30 and require life-long treatment.
Cannabidiol is a non-psychotropic derivative of cannabis, which has demonstrated therapeutic effects for serious neurological conditions including rare seizure disorders, and for alleviating symptoms of multiple sclerosis.
VBIO‘s prodrugs — drug converted into their effective form after processed in the body — could exert the same beneficial therapeutic effects, but with notable improvements like a better tasting formulation, better oral bioavailability, or a delayed release mechanism which enables patients using these medications to enjoy long-lasting, overnight relief.
VBIO is taking aim at two areas in particular… multiple sclerosis and inflammatory bowel disease.
Check out this Vitality Cannabinoid Video for further proof
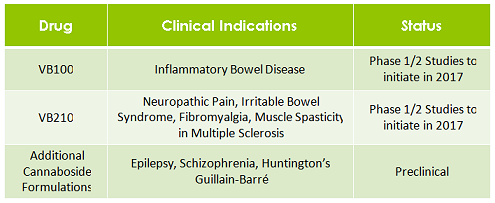
VBIO’s VB100 drug has been developed as a treatment for IBD.
VBIO is conducting the first-in-man clinical studies of proprietary cannabinoid glycoside ‘cannabosides’ as a therapy for Inflammatory Bowel Disease.
VBIO’s VB210 will seek to relieve symptoms of multiple sclerosis as well as address similar indications like fibromyalgia and neuropathic pain. Both drugs are expected to start their phase 1/2 studies in 2017.
VBIO’s newest drug is in preclinical development, and VBIO has more than 40 pending patents on other cannabinoid-based glycoside prodrugs.

Simply put, Prodrugs are medications or compounds that are converted within the body into a pharmacologically active drug
Because the reference drug already has independent verification of its safety and efficacy, the prodrug may be approved rapidly through demonstrating similar bioavailability or bioequivalence, and at the same time a prodrug can be far more marketable due to its ability to eliminate unwanted side effects or undesirable commercial aspects.
There is one ProDrug you’ve been using your entire life: ASPIRIN
Aspirin, also known as acetylsalicylic acid, was first made by Felix Hoffmann at Bayer in 1897 and is a synthetic prodrug of salicylic acid.
As of 2015, there were approximately 15 prodrugs that had been classified as blockbusters, defined as having annual sales in excess of $1 billion.
Robert Brooke, VBIO CEO, recently shed new light on key factors in the fight against the increasing epidemic of prescription painkiller related deaths.
In a recent article, Brooke states, “Opiates are one of the key classes of drugs we’re seeking to replace, or to make far less necessary, as our proprietary cannabosides could provide a potent alternative form of pain relief and help avoid, or greatly reduce, the use of opiates for the treatment of many conditions including inflammatory bowel disease (where 70% of patients now regularly receive opiates upon hospitalisation).”
The statistics are startling, as last year there were 47,055 Americans that died from drug overdoses, and opioids were involved in 61% of these cases. Since 2013, the rates of drug-overdose deaths have exceeded the number of deaths from car accidents. This is not uniquely an American problem, but the U.S. uses an astonishing 80% of the world’s opioids, while representing only 4.6% of the world’s population. The New England Journal of Medicine has just written that the rising death toll has been rivaled in modern history only by that at the peak of the AIDS epidemic in the early 1990s.
Opiates are incredibly potent pain relievers. They are a panacea for patients with severe traumatic pain or dealing with end-of-life care. But in recent years there has been widespread use and abuse of them. There has been a 10-fold increase in the medical use of opioids in the last 20 years in a movement towards aggressive management of pain. Opiate addiction is in the news again recently, with the death of famed musician Prince linked to fentanyl, and reports from Cincinnati where 174 heroin overdoses occurred in 6 days, when street heroin was mixed with powerful synthetic opiates like fentanyl and carfentanil. More than 2.4 million Americans now have a severe opioid-use disorder, which includes dependence on heroin and also prescription opiate drugs that are known by many names including fentanyl, morphine, hydrocodone, OxyContin, Vicodin, Codeine, Percocet, and illicit “street” versions of these same drugs.
The incidence of heroin initiation is 19 times higher among those who report prior abuse of pain medications (i.e. a “gateway” drug). Studies have shown that today about 75% to 86% of heroin users started with prescription opioid pain relievers. So the first exposure to opiates is far more likely to come not from heroin (as was overwhelmingly true in the 1960’s), but rather from prescription drugs.
Beyond that, there’s startling evidence that opiate addiction may often originate simply by following your doctor’s orders, i.e. not from prescription drug abuse, where the prescription drugs were obtained illegally or misused, but through addiction after receiving and following a legitimate prescription. A 2012 Canadian study showed that 22% of people who were prescribed opioids by their doctors for long-term pain management started abusing the pills. Especially for people at high genetic risk of addiction, giving them even a short dose of prescription opiate medications may be too much for them to handle, like giving them a loaded gun without any training or warning. Doctors are increasingly aware of this, and some are pleading with their physician colleagues to face reality. Think about that the next time your doctor gives you or your child a seemingly harmless Vicodin, Percocet, or Codeine prescription after a minor surgery.
Both the medical community and the pharmaceutical industry know there’s a desperate and (also never ending) need for safe and effective pain relief — 40% of older adults live with chronic pain. But the rampant abuse of opiates and their questionable efficacy for chronic pain means they are not the answer. It’s a significant reason why VBIO’s work on cannabinoids is so important. Cannabinoids are already starting to deliver, as clinical studies in IBD have already shown that they can help patients to be weaned off narcotics and corticosteroids. Cannabosides could provide these same therapeutic benefits and more. Through site-specific delivery they can also avoid the psycho-activity and sedation of THC. There is plenty of irony in developing pharmaceuticals derived from what remains an illicit drug by the federal government. That said, this is a drug, and VBIO’s pharmaceutical solution in particular, where overdoses are near impossible and where the medical community is strongly turning in its favor.
VBIO’s science provides for several advantages. It allows for several benefits which other cannabinoid, non-prodrug treatments don’t offer. Specifically, since cannabidiol doesn’t create a psychoactive effect, VBIO’s drugs allow for higher, more effective dosing. Since these are prodrugs and aren’t activated until in the body, they allow for targeted delivery to the GI tract (yet can also selectively target other body parts, even as far away from the GI tract as the brain). Perhaps more than anything though, these drugs are orally-administered by virtue of being highly soluble, making them easy to use.
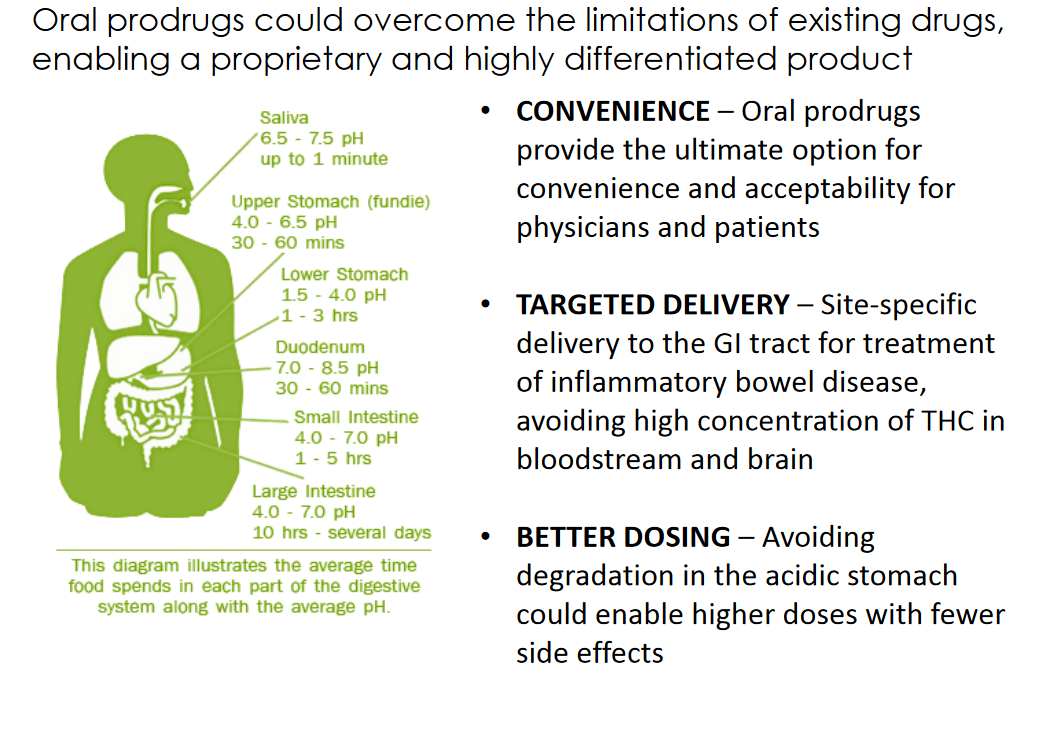
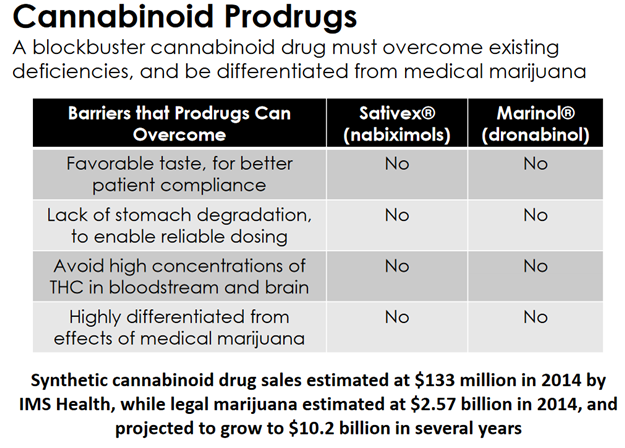
As impressive as some other cannabinoid drugs have been, their use has been known to cause significant problems like hives (when delivered with a transdermal patch) or oral lesions (when used with an alcohol-based mouth spray).
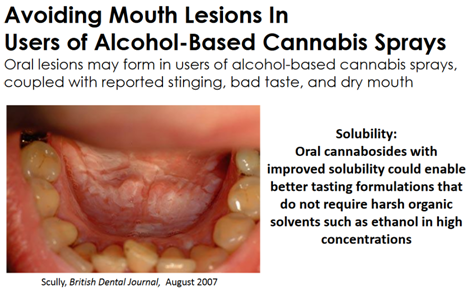
By comparison, the cannabinoid drug Sativex from GW Pharmaceuticals (GWPH) and Marinol from AbbVie (ABBV) cannot prevent the side effects of these types of therapies. Vitality Biopharma’s (VBIO) drugs do.
It stands to note that while GWPH ($3.3 Billion Market Cap) and ABBV ($102.5 Billion Market Cap) have already experienced historic runs, VBIO still remains relatively unnoticed with a market cap still under $18.0 Million (as of October 8, 2016).
Another reason one might want to take a speculative position in VBIO: Vitality isn’t trying to reinvent the wheel.
The industry has already proven cannabis-based drugs can be effective, particularly as therapies for inflammatory bowel disease, muscle spasticity in multiple sclerosis, neuropathic pain, schizophrenia, and even control of seizures. VBIO is simply building on proven research with an even better version of existing therapeutic approaches. That reality lowers the risk of an investment by upping the odds of success.
And make no mistake – cannabinoid drugs are already a proven market. Synthetic cannabinoid drug sales were estimated at $133 million in 2014, and that was before regulators became at least semi-friendly to the idea. Just think what could happen when VBIO explains to regulators how its drugs are non-psychoactive.
The multiple sclerosis market is worth approximately $18 billion per year, and yet their needs are seriously unmet.

The Inflammatory Bowel Disease market is worth an estimated $7 billion in the U.S. alone, and is approaching $10 billion on a global basis. As for cannabinoids, some believe this budding market — now that governments are more accepting of them — could exceed $20 billion by 2020.
If VBIO can penetrate even a fraction of that market, these are monster numbers – especially when compared to the miniscule market cap of the company.

VBIO looks to be in a great position right now. With the US Presidential elections just a month away, Marijuana and Cannabis/Cannabinoid stocks are on the receiving end of tremendous buy-side volume. With legislation set to pass on both the state and federal levels, this opens the doors for companies like Vitality Biopharma to benefit you as both a consumer and shareholder.
Get started on your research now.

