Potential for FDA Fast-Track Approval Designation & Imminent Product Launch
Pure Pharmaceutical Cannabidiol, Cardiol Therapeutics Inc.’s (TSX:CRDL)(OTCQX:CRTPF) Medicine has a Significant First-to-Market Advantage
When it comes to pharmaceuticals, timing is EVERYTHING.
The 2010s were defined as a decade of drug breakthroughs. Over the last 10 years, the Nasdaq Biotech Index is up 355% from 843 to 3,000 (this is even after the coronavirus crash!).
Among the top 10 performing stocks of 2019, an incredible SIX were pharma companies… including four of the top five.[1]
Even with this coronavirus market crash, many biotech stocks have been flying. There’s always a disease or condition that is looking to be treated.
Many successes came from the pursuit to discover a breakthrough treatment. But there also came a surge of innovation for developing products for rare diseases and conditions, thanks to added incentives put forth by the FDA[2], and a Global Orphan Drugs Market that is expected to generate $169 billion in revenue by 2022.[3]
Drugs that are being developed to treat rare diseases (defined in the US as those affecting less than 200,000 patients) can take advantage of the FDA’s Office of Orphan Products Development (or Orphan Drugs)[4] incentives and enjoy additional benefits as well as being first to market.
In particular, orphan drug developers get an expedited approval process, which saves millions of dollars in drug development costs and provides a period of seven to nine years of exclusive access to the market.
Once successful, companies granted orphan status for their drugs have a very substantial profit window.
Press Releases
- Cardiol Therapeutics Signs Supplier Agreement with Medical Cannabis by Shoppers, a Subsidiary of Shoppers Drug Mart Inc.
- Cardiol Therapeutics Receives Health Canada Approval for Phase 1 Study of its Pharmaceutically Produced Formulation
- Cardiol Therapeutics’ Exclusive Manufacturing Partner Receives Three-Year Renewal and Amendment of its Cannabis Act License from Health Canada
- Cardiol Therapeutics Appoints Michael J. Willner, Esq. as Business Advisor
- Cardiol Therapeutics Appoints Former GW Pharmaceuticals Executive Colin Stott to its Board of Directors
Cardiol Therapeutics Has Major Near-Term Catalyst Right Around the Corner
Last June, Cardiol Therapeutics (TSX:CRDL)(OTCQX:CRTPF), a still under-the-radar specialty pharmaceutical developer, announced its plans for an orphan drug program in acute myocarditis with its CardiolRx™ CBD formulation.
Acute myocarditis is an inflammatory condition of the heart most often resulting from viral infection and is a major cause of sudden death in children and young adults. Plans for a Phase 2 international trial of CardiolRx™ in acute myocarditis, to be conducted at world leading centers of excellence for heart research, were announced in November.

The potential benefits of CBD on myocarditis have been studied experimentally and have attracted major medical interest over the past few years.[5],[6]
The Cardiol Therapeutics (TSX:CRDL)(OTC:CRTPF) team consists of winners from within the pharmaceutical sector, including a President and CEO who previously founded and grew a company to a $1 billion valuation, and a legendary Chief Medical Officer who’s received the Order of Canada among many other accolades.
Now there is the potential for CardiolRxTM to become the next cannabidiol product to be approved and the first one that is synthetically derived, 99.9% pure, and THC free (<5ppm).
This has analysts watching Cardiol Therapeutics (TSX:CRDL)(OTC:CRTPF) with keen interest.
Cardiol’s analyst coverage includes:
With its industry-leading product purity levels and accomplished management team, Cardiol Therapeutics (TSX:CRDL)(OTC:CRTPF) is in the process of making a strong case to potentially gain the approval of both Health Canada and the US FDA for CardiolRx™—a feat that could potentially yield them that coveted first-to-market advantage.
Cardiol Therapeutics – A Pharmaceutical Cannabidiol Company from Cardiol Therapeutics on Vimeo.
Purity Levels That Far Outpace Peers
Perhaps where Cardiol Therapeutics (TSX:CRDL)(OTCQX:CRTPF) excels the most is in the purity of its product—namely, its extremely low levels of THC.
This means the product is completely non-intoxicating.
It’s worth noting that in 2018, the US FDA approved the first drug comprising an active ingredient derived from cannabis.[7]
This drug treats rare, severe forms of epilepsy, and is produced by GW Pharmaceuticals, plc (NASDAQ:GWPH).
Note: Cardiol recently appointed former GW Pharmaceuticals Scientific Affairs Director, International and R&D Operations Director, Colin Stott, to its Board of Directors.
Purity matters, as even small traces of THC may possibly yield THC-positive urine drug tests.
The US government defines CBD oil as any extract containing 0.3% or 3000ppm THC or less.
By comparison, Cardiol’s high-purity product goes far beyond the standard, with levels even less than GW Pharmaceuticals’ FDA approved medicine for rare forms of paediatric epilepsy.
Comparable Purity Levels for High Concentration Cannabidiol Formulations
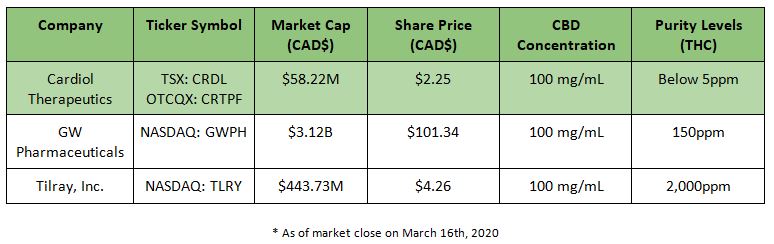
It’s clear that Cardiol Therapeutics’ CardiolRxTM has an ultra-high purity, (<5ppm THC), that sets a new purity standard for the industry.
This unique product profile should offer a significant competitive advantage when it comes to future drug approvals and meeting the growing demands from physicians, patients, consumers, and regulators for consistent, high quality, high purity products.
Cardiol Therapeutics Launching Commercial Sales While Advancing its Clinical Program
Looking ahead in 2020, Cardiol Therapeutics (TSX:CRDL)(OTCQX:CRTPF) is preparing to launch its flagship commercial product, CardiolRx™, as the purest high-concentration cGMP manufactured pharmaceutical CBD oil alternative product in the world.
CardiolRx™ will be launched imminently in the growing $1.2 billion medicinal hemp oil market in Canada, while in parallel the Company prepares to initiate its clinical development programs in Canada, the US, and Europe targeting life-threatening heart disease.
Back in 2018, another drug, Epidiolex®, which contains ultra purified CBD as its only active ingredient became the first ever FDA-approved drug that contains a purified active ingredient derived from cannabis.
Now there is the potential for Cardiol’s CardiolRx™ to become the first pharmaceutically produced product to be commercialized that contains the identical active ingredient found in Epidiolex® at the exact same concentration. This ultra-pure (<5ppm THC) product has analysts watching Cardiol Therapeutics (TSX:CRDL)(OTCQX:CRTPF) with keen interest.
A Standout Biotechnology Play
What truly makes Cardiol Therapeutics (TSX:CRDL)(OTCQX:CRTPF) stand out as a leader with a strong competitive advantage is its unparalleled supply chain to pharmaceutically produce commercial scale volumes of a cannabidiol commercial product that will be available to consumers in early 2020.
Through its exclusive partnerships with one of the world’s largest pharmaceutical manufacturers of controlled substances, Noramco, Inc., (through Purisys, LLC, an affiliate of Noramco) and one of the most experienced pharmaceutical formulators of controlled substances, Dalton Pharma Services, Cardiol Therapeutics has created an ultra-pure/THC free product.
Currently, the Canadian medicinal cannabinoid market is valued at an estimated $1.2 billion, but patients under 25 and over 65 are underserved. Cardiol has created a premium, ultra-pure  product to address the needs of these patient populations where there is little to no competition.
product to address the needs of these patient populations where there is little to no competition.
CardiolRx™ has been developed with <5ppm THC. This product provides a distinct competitive advantage for Cardiol as it addresses the growing demand from patients who should not be exposed to THC; essentially, anyone under the age of 25 and many over the age of 65. These age groups represent large underserved segments of the growing $1.2 billion Canadian medical hemp oil market.
Physicians have been cautioned by the Canadian Paediatric Society that exposure to THC before the age of 25 can lead to unknown effects on brain development. In older patients, side effects of exposure can lead to unfavorable drug interactions and intoxication.
The company just received the green light from Health Canada to proceed with a Phase 1 study of its pharmaceutically produced (cGMP), high concentration, pure CBD oil formulation.
This is a huge milestone for Cardiol Therapeutics (TSX:CRDL)(OTCQX:CRTPF) and for the industry as a whole, as it’s the first-ever Canadian clinical trial of a pharmaceutically produced CBD oil formulation that is specifically designed for patients who should not be exposed to THC.
The Phase 1 study, which the company expects to start in Q2, 2020, is designed to measure the pharmacokinetics (blood levels of drug) following both single and multiple doses of the high concentration, pure CBD oil formulation in up to 55 healthy subjects, both in the fasting and fed states.
The study will also measure standard safety parameters at escalating doses to help select the optimal dosing levels for the company’s planned international Phase 2 study in acute
Health Canada First, then on to the FDA
Cardiol Therapeutics’ (TSX:CRDL)(OTCQX:CRTPF) ultra-pure formulation is pharmaceutically produced specifically for medical purposes.
With less than 5ppm THC, this pharmaceutically produced cannabidiol is one of the purest in the world; the level is below Health Canada’s threshold for requiring manufacturers to place a THC warning label on product packaging.
But it has so much more going for it.
It’s free from side effects of THC.
It’s produced using controlled processes at a cGMP, Health Canada approved, FDA registered and inspected pharmaceutical company.
This allows for consistent quality and purity, resulting in a pure (<5ppm THC) oil—ideal for patients who should not be exposed to THC.
Orphan Drug Candidate Entering Clinical Trials and Mass Market Nanoformulation in Development
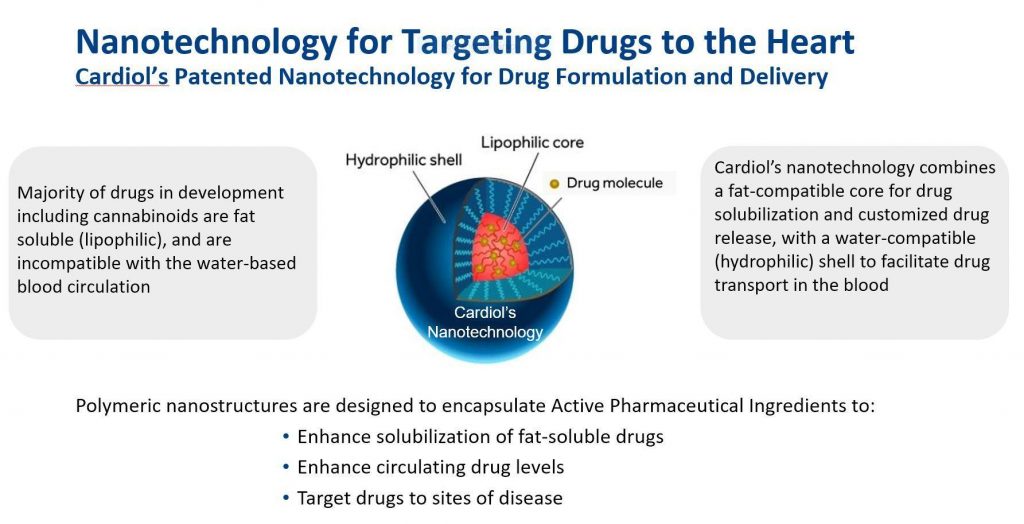
Cardiol has engaged an international research network to develop novel pharmaceutically produced CBD oil medicines to target the treatment of heart failure. There have been no significant treatment advances in diastolic heart failure in over 20 years. Over six million adults in Canada and the United States suffer from chronic heart failure and it is the leading cause of death and hospitalization in North America, with associated annual healthcare costs in the U.S. alone exceeding $30 billion.
Cardiol Therapeutics’ (TSX:CRDL)(OTCQX:CRTPF) ongoing research program has shown the potential of the company’s patented nanoformulation of cannabidiol at the prestigious Houston Methodist DeBakey Heart & Vascular Center, Texas, the home of the first heart transplant performed in the United States. The Houston data demonstrated the ability of the company’s nanotechnology to target CardiolRx™ to sites of inflammation and fibrosis in diseased hearts.
World-Class Partnerships
Cardiol Therapeutics Inc. (TSX:CRDL)(OTCQX:CRTPF) is not going into this alone. The company has wisely entered into world-class partnerships, enabling it to meet global demand through unparalleled access to cGMP manufacturing of a pharmaceutically produced CBD oil.
1 – Dalton Pharma Services – Health Canada-approved, FDA-registered, cGMP manufacturer of pharmaceutical cannabidiol products
Cardiol has an exclusive global manufacturing agreement with Dalton for the supply of cGMP manufactured pharmaceutical CBD oil products for commercial introduction. Dalton recently received a three-year renewal and amendment of its Health Canada license.
 2 – Noramco, Inc. – Global leader in the manufacture and supply of controlled drug substance APIs
2 – Noramco, Inc. – Global leader in the manufacture and supply of controlled drug substance APIs
Cardiol has an exclusive agreement with Noramco (through Purisys, LLC, an affiliate of Noramco) for the manufacture and supply of a pure active pharmaceutical ingredient found in CBD oil for Canada and Mexico.
3 – TecSalud del Tecnológico de Monterrey – Latin America’s Largest Private Research Network
Cardiol is working with TecSalud to develop the scientific background to support clinical trials. TecSalud has made a US$3,000,000 investment commitment in Cardiol to support nanotherapeutics research and is collaborating with leading heart centers, including the Houston Methodist DeBakey Heart & Vascular Center, and Massachusetts Institute of Technology (MIT).
Superstar Management Team and Board Leading the Way




7 Reasons
to Further Examine Cardiol Therapeutics (TSX:CRDL)(OTCQX:CRTPF)
1
Imminent launch of the world’s purest, THC free (<5ppm) CBD oil alternative product puts Cardiol’s CardiolRx™ in a league of its own.
2
Initiating an orphan drug eligible program in acute myocarditis; a heart condition that represents a leading cause of sudden death in people under the age of 35.
3
Advancing a potential breakthrough therapy for heart failure; a leading cause of death and disability in North America with associated annual healthcare costs exceeding US$30 billion.
4
Established partnerships with world-class pharmaceutical companies to support large scale manufacture and distribution.
5
Enter into commercial agreements for product launches in North America, Latin America, Australasia, and Europe.
6
Exceptional research partnerships with leading institutions and health centres across North America and Europe.
7
Superstar leadership team with proven track record of company building and growing market caps over $1 billion.
[1] https://qz.com/1778006/pharma-stocks-topped-the-list-of-top-performers-in-2019/
[2] https://www.fda.gov/industry/developing-products-rare-diseases-conditions
[3] https://www.alliedmarketresearch.com/orphan-drug-market
[4] https://www.fda.gov/industry/developing-products-rare-diseases-conditions
[5] https://jhu.pure.elsevier.com/en/publications/cannabidiol-limits-t-cellmediated-chronic-autoimmune-myocarditis-
[6] https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3579247/
[7] https://www.fda.gov/news-events/press-announcements/fda-approves-first-drug-comprised-active-ingredient-derived-marijuana-treat-rare-severe-forms
Disclosure:
Disclaimer: This release/advertorial is a commercial advertisement and is for general information purposes only. This is a Native Advertisement, meaning it is an informational paid marketing piece. WallStreetNation.com makes no recommendation that the securities of the companies profiled or discussed on this website should be purchased, sold or held by viewers that learn of the profiled companies through our website. Please review all investment decisions with a licensed investment advisor. This Advertorial was paid for by the issuer in an effort to enhance public awareness of Cardiol Therapeutics and its securities. DF Media has or expects to receive over one hundred thousand dollars by the issuer as a total budget for this advertising effort on CPC campaign. Neither WallStreetNation.com or DF Media currently holds the securities of Cardiol Therapeutics and does not currently intend to purchase such securities. This Advertorial contains forward-looking statements that involve risks and uncertainties. This Advertorial contains or incorporates by reference forward-looking statements, including certain information with respect to plans and strategies of the featured Company. As such, any statements contained herein or incorporated herein by reference that are not statements of historical fact may be deemed to be forward-looking statements. Without limiting the foregoing, the words “believe(s)” “anticipate(s)”, “plan(s)” “expect(s)” “project(s)” “will” “make” “told” and similar expressions are intended to identify forward-looking statements. There are a number of important factors that could cau se actual events or actual results of the Company to differ materially from these indicated by such forward-looking statements. Certain statements contained herein constitute forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and 21E of the Exchange Act of 1934. Such statements include, without limitation, statements regarding business, financing, business trends, future operating revenues and expenses. There can be no assurance that such expectations will prove to be correct. Investors are cautioned that any forward-looking statements made by the Company, or contained in this advertorial are not guarantees of future performance, and that the Issuer’s actual results may differ materially from those set forth in the forward-looking statements. Difference in results can be caused by various factors including, but not limited to, the Company’s ability to be able to successfully complete planned funding agreements, to successfully market its products in competitive industries or to effectively implement its business plan or strategies. To reiterate, information presented in this advertorial contains “forward-looking statements”. Any statements that express or involve discussions with respect to predictions, expectations, beliefs, plans, projections, objectives, goals, assumptions, or future events or performance are not statements of historical fact and may be “forward-looking statements.” Forward-looking statements are based on expectations, estimates, and projections at the time the statements are made that involve a number of risks and uncertainties which could cause actual results or events to differ materially from those presently anticipated. Forward-looking statements in this advertorial may be identified through the use of words such as “expects,” “will,” “anticipates,” “estimates,” “believes,” “may,” or by statements indicating certain actions “may,” “could,” or “might” occur. More information on the Company may be found at www.sec.gov readers can review all public filings by the Company at the SEC’s EDGAR page. WallStreetNation.com is not a certified financial analyst or licensed in the securities industry in any manner. The information in this Advertorial is subjective opinion and may not be complete, accurate or current and was paid for, so this could create a conflict of interest.


