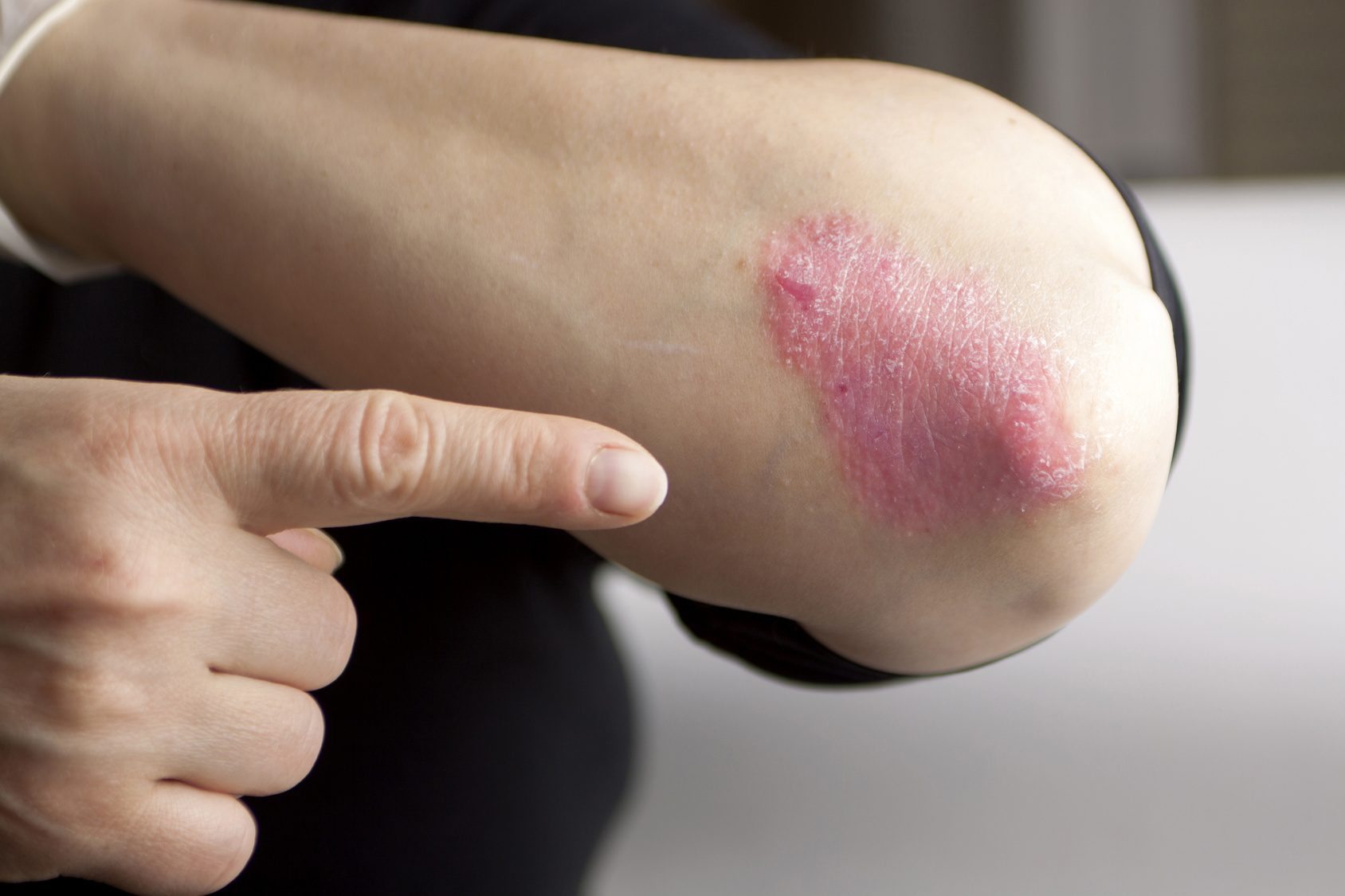
This New Drug Could Help Psoriasis Sufferers
Between 2 to 4 percent of the population suffers from Psoriasis and those who have it, know just how debilitating it can be mentally and physically. Psoriasis is believed to be caused by abnormal production of skin cells at accelerated rates which then results in red skin patches.
Ixekixumab is a new drug that is looking to be highly effective in treating moderate to severe psoriasis in a majority of patients. These findings come from three pivotal clinical trials assessing Ixekizumab.
Developed by Eli Lilly and recently approved by the U.S. Food and Drug Administration (FDA) since the completion of these trials, Ixekizumab (brand name, Taltz) is a humanized monoclonal antibody that neutralizes the pathway promoting the development of psoriasis.
The findings, which led to the drug’s approval, are detailed in the study “Phase 3 Trials of Ixekizumab in Moderate-to-Severe Plaque Psoriasis,” published in the New England Journal of Medicine.
In the trials, a total of 3,736 adult patients with moderate to severe psoriasis from 21 countries, were randomly assigned to either receive injections of varying doses of Ixekizumab or to placebo for slightly more than one year.
After 12 weeks of treatment, results showed that between 76.4 percent and 81.8 percent of the psoriasis patients had “clear” or “minimal” disease compared with only 3.2 percent of those on placebo. Moreover, the majority of patients (68.7 to 78.3 percent) maintained their “clear” or “minimal” disease status at week 60 of treatment.
Monitoring will be needed for patients taking the drug for more than 60 weeks as there were reported side effects in the patients receiving ixekizumab. This included reduced white blood cell counts, yeast infection, and inflammatory bowel disease when compared to the placebo group.